Camphene: Tracing Its Practical Roots and Present-Day Promise
A Look Back: Camphene’s Road Through History
Before electricity’s hum filled city nights, camphene already stood shoulder to shoulder with whale oil, lighting homes across nineteenth-century America and Europe. People found it cost less, came from pine resin rather than whales, and kindled well enough to challenge pricier lamp fuels. Old records from the 1840s mention its widespread use, especially before kerosene took center stage. Its sharp scent and bright, clear flame made it part of everyday life from rural cabins to urban parlors. Behind that familiar aroma—which gardeners and mechanics still recognize—camphene holds a history woven with industry, resourcefulness, and shifting science.
The Goods: A Hands-On Overview of Camphene
Today’s camphene usually appears as a colorless, crystalline solid that gives off a pungent, pine-like odor. Folks in perfumery, pesticide formulation, and even polymer manufacturing come across it as both a raw material and a specialty intermediate. Its main use shifted over the years, moving from light source to chemical niche. Camphene arrives to market in drums or bags, packed with purity guarantees and usually labeled with its standard chemical identifier, C10H16, matching its terpene cousins.
Diving Into Its Character: Physical and Chemical Properties
Camphene melts close to 50°C, boils up at 160°C, and dissolves just enough in alcohol or ether to play well in assorted blends, but shrugs off water almost completely. Fire risk comes from its low flash point, around 34°C, so workers treat it with care around sparks or open flames. Structurally, it fits into the terpene family as a bicyclic monoterpene; this ring structure helps explain its reactivity and aroma. Its molecular setup makes it eager to tumble through organic reactions, whether that’s taking on halogens, rearranging with acid, or acting as a building block for more elaborate compounds.
Camphene Specs: Detailed Labeling and Character
A standard label on a can of pure camphene announces its CAS number, clear limits on water content—usually below 0.5%—and a guarantee of purity, commonly quoting figures above 95%. Bottles often specify melting and boiling points for laboratory reference, along with warnings for flammability and health hazards. For large-scale buyers, suppliers share certificates spelling out everything from optical rotation to residual solvents, making sure the material lines up with chemical and industrial rules in Europe, North America, and Asia.
Making Camphene: Preparation in the Real World
Manufacturers tend to kick off camphene production with turpentine oil, a familiar distillate from pine resin. They run it through a catalytic isomerization process, often relying on strong acids or clays as catalysts, which coax the α-pinene in turpentine into giving up its structure and twisting into camphene’s form. Industry notes point out that acid catalysts—like sulfuric acid—work efficiently, but crews stay on guard for side products. Once reactors finish their work, distillation sorts out the camphene, often leaving behind byproducts that still find use in solvents or adhesives.
Chemical Interactions and Pathways to New Uses
Camphene’s structure gives it opportunities to morph into other chemicals or add fresh branches to its network. Oxidizing agents can flip it into camphor, while hydrogenation turns it into isobornyl derivatives, which perfumers and cleaning-product designers study with interest. Researchers have reported halogenation and nitration yielding specialty intermediates for agrochemical synthesis. Those transformations draw both academic attention and industry dollars, especially anytime a process slashes costs, waste, or hazard. The world of green chemistry chases catalysts that trim energy demands, aiming for lower emissions and smaller footprints, a trend that’s gaining momentum year by year.
Names on the Label: Synonyms and Trade Monikers
Alongside “camphene,” people in chemistry labs or oil refineries might see labels like “bornylene” or reference numbers such as FEMA 2230 used in fragrance listings. Scientific texts stick with “2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane,” though few people outside technical circles use that mouthful. European and Asian suppliers sometimes opt for regional identifiers, but the camphene name usually keeps top billing, especially in import and export documents.
Health and Safety: Protocols Grounded in Experience
Anyone who’s handled camphene in bulk knows it requires respect and solid training. It burns fiercely, so storage spaces need good ventilation and strict spark controls. Guidelines call for gloves and goggles during handling, with clear instructions for spill cleanup or accidental ingestion. MSDS forms show hazard codes for its flammable and mild irritant properties. In jobs where camphene dust might hang in the air, extraction fans and masks keep the workforce safe. Industrial safety standards, whether from OSHA or European agencies, apply strict labeling and training—especially for labs, shipping crews, and facilities bordering residential zones.
Camphene at Work: From Chemistry Bench to Industry Floor
Forestry engineers and chemists put camphene to use all across manufacturing. Soap makers blend it for fragrance, using its crisp, piney aroma. Paint technicians add it to thinners and varnishes, counting on its volatility and solvency. Some food companies use minuscule, regulated amounts of camphene for flavoring, reflecting its spot on FEMA’s sweetener and perfume registers. In insecticide labs, researchers turn to camphene as a building block for pyrethroid synthesis. Even the plastics world looks at camphene derivatives to modify polymers, playing with toughness and scent. Each industry shades its standards, but the reliance on camphene’s traits crops up wherever sharp scent or reactive terpenes fit the job.
New Horizons: Research and Development Chasing Innovation
Synthetic chemists continually probe camphene’s reactivity, hoping to carve out new families of fine chemicals or more efficient catalysts. Biochemists trace its environmental impact, especially where turpentine extraction crosses paths with conservation. Recent years saw teams exploring camphene’s anti-inflammatory properties in cell models, eyeing its potential in botanical medicines or as a pesticide with lower aquatic toxicity. Organic chemists run experiments tweaking camphene’s skeleton to yield new monomers, resins, and even more potent fragrances, tying the molecule’s fate to wider biotech advances. Every breakthrough shifts the baseline for cost, quality, or sustainability, keeping camphene’s story evolving.
Toxicity Research: Honest Lessons From the Lab
Toxicologists report that camphene has low acute toxicity by oral or dermal routes, though its flammability brings serious fire risks if mishandled. Studies on rodents show effects only at high doses, with most regulatory agencies rating it safe for low-level use in approved contexts. Skin and eye irritation crops up for workers with repeated exposure; proper gloves and face protection solve most problems. Environmental studies run tests on aquatic life, flagging toxicity only at concentrated discharges—well above levels seen from field use. Public health agencies keep a close watch on inhalation risks, tweaking exposure limits in workplaces based on ongoing health surveillance.
Prospects: Where Camphene’s Path May Lead
The future for camphene stands open. With more pressure on sustainable sourcing, industries focus on extracting it from plantation pine and processing it with catalysts that sip rather than gulp energy. Ongoing research may bump up its value in pharmaceuticals, green solvents, or as a launching point for biodegradable plastics. Demand may spike if perfumers and cleaners keep chasing sharper, more “natural” scents. New safety guidelines, built from real-world data, should sharpen best practices in manufacturing and transport. Whether biotech can turn camphene into a springboard for advanced renewable chemicals remains a question worth following. Its balance of scent, reactivity, and resource roots anchors its relevance in both science’s labs and industry’s loading docks.
Understanding Camphene in Everyday Life
Walking through a hardware store or reading labels on household items, camphene doesn't jump out as a celebrity chemical. Yet, it fills a quiet but important place in industry and our homes. Derived from turpentine oil, camphene carries a pine-like scent and a long history dating back to the 19th century, where it once served as a lamp fuel before kerosene took the stage. The substance might have faded from the spotlight, but its role hasn’t evaporated.
Camphene in Fragrance and Flavoring
My experience working in a botanical processing plant gave me direct exposure to how industries use aromatic compounds. Camphene’s crisp, woody smell makes it hard to ignore for anyone mixing fragrances or preparing flavoring agents. Manufacturers add it to soaps, detergents, and even air fresheners. In food, it gets used as a flavoring component, though in much lower amounts. Its distinctive scent offers a recognizable signature, helping create the right aroma for products that need a touch of nature without relying on artificial notes.
Solvents and Specialty Chemicals
Camphene doesn’t just provide scents; it also serves as a handy building block in chemical manufacturing. Making synthetic camphor, for example, involves camphene. Synthetic camphor finds its way into medicinal balms, liniments, and even some food packaging. Living in a part of the country where cold weather aches make menthol and camphor balms a medicine cabinet staple, I notice how reliant the health industry becomes on these chemical intermediates.
Paints, varnishes, and lacquers use camphene, too. Its volatility helps paints dry faster. Companies find a certain advantage in using it for coatings: camphene's quick evaporation cuts down on the sticky wait and supports a cleaner finish. Smaller manufacturers, especially those crafting specialty paints, rely on its properties to achieve the consistency customers expect.
Insect Repellent and Agriculture
Not too long ago, I spent a season volunteering with an organic gardening project. Some natural pesticides listed camphene as an ingredient. Scientific papers back up its role in various essential oil blends used to deter insects in grain storage or crop fields. Agricultural specialists search for alternatives to harsh synthetic chemicals, and camphene’s origins from pine and fir oils make it attractive for those who want greener methods.
Health and Safety: Points to Remember
Any time a chemical shows up in personal products, safety becomes a concern. Camphene has caught the attention of regulatory agencies, which limit concentrations in consumables and cosmetics. Workers exposed to high levels, like those in flavoring or solvent plants, wear protective gear because inhalation or skin contact in large amounts can irritate lungs or skin. Makers push for better ventilation and handling practices, but smaller businesses sometimes lag in implementing these safety upgrades.
What’s Ahead for Camphene?
People look for greener chemicals—something not derived from petroleum, less persistent in the environment. Camphene hits a few marks: It comes from renewable sources such as pine trees and fits into natural product formulations. Research into improved extraction from plant sources, better workplace safety rules, and safer blending in household goods could all refine its niche use. As people demand more transparency and sustainability, camphene’s roots in nature could win it a stronger position in consumer products.
Understanding Camphene
Camphene rolls off the tongue like a character out of a chemistry class, yet it’s actually a terpene hiding in the scents of pine, turpentine, and ginger. Manufacturers rely on camphene to produce fragrant oils and flavoring agents, so traces show up in some foods and cosmetics. More biohacking brands and supplement companies have started exploring terpenes, too. A person might wonder, should this compound land on our daily menu?
Potential Risks and Scientific Takeaways
A quick look at toxicology studies gives the impression camphene won’t kill you on contact. Toxicologists gave it to lab rats, and those rats lived to tell the tale—at least in small doses. One study flagged modest liver stress at higher intakes, which throws a little caution to the wind. The Food and Drug Administration classifies camphene as “Generally Recognized As Safe” for use in food, provided it stays within low, typical amounts.
You don't usually see people chugging pints of essential oils or eating spoonfuls of chemicals from the lab, so actual exposure from real food tends to sit far below any alarm-raising threshold. That’s some peace of mind. But I do believe that stuffing anything into groceries just because test animals don’t drop dead can skirt personal responsibility. Even regulatory bodies admit that “safe” status depends on both the dose and the form, and there’s a difference between a minor aromatic trace and an unsupervised supplement dosage.
What Real-World Experience Shows
Working with food flavoring in my own kitchen, I learned early to respect the power concentration brings. A drop of camphene oil can infuse a batch of hard candy with an almost medicinal, resinous kick. Using more? The entire pan starts smelling like a hardware store aisle. Strong flavors and scents don’t take much to overpower a recipe—or a stomach. I've tasted enough botched experiments to know when a molecule oversteps into excess, the body responds with a warning.
Friends with allergies or chemical sensitivity often notice headaches or nausea around synthetic fragrances or pungent essential oils. Camphene fits that pattern. It irritates sensitive skin, and its vapors—found in some air fresheners—don’t do asthmatics any favors. That earned it a spot on hazard lists for inhalation and dermal exposure, which matters for folks who tinker with essential oils at home.
Responsible Solutions
Manufacturers should stick to evidence-backed doses when adding camphene to any food or supplement. Food scientists and product formulators ought to label ingredients clearly and avoid “whole plant” or “natural blend” phrasing if camphene stands out. Every consumer deserves to know exactly what they’re chewing or breathing in. Curiosity-driven supplement companies can earn trust through transparent sourcing, third-party testing, and clear warnings for people with respiratory sensitivities or allergies.
As people chase novel wellness trends, it pays to remember that safety depends on both science and common sense. Reach for natural flavors and terpenes, sure, but don’t skip the basics of moderation and proper labeling. Relying on animal studies or past regulatory green lights only goes so far; we've seen enough surprising side effects crop up when science meets real-world diversity in human health. In any case, camphene isn’t some toxic invader, but it does call for some respect—especially from anyone who’s tempted to turn a dash into a dose.
Understanding What Happens in Real Life
Camphene, a terpene you find in a lot of essential oils, keeps popping up in products at the local natural health store. It’s there to offer fragrance, and gets promoted for its supposed health perks, from soothing coughs to jazzing up your lungs. I’ve seen folks make their own topical creams with it, and others swear by camphene’s clearing aroma in the diffuser. But it’s not all smooth sailing. Personal experience and medical reports both throw up some warnings you can’t ignore.
Why Side Effects Matter—Personal Experience and Facts
After hearing about camphene’s “natural” origin, people sometimes treat it like it’s harmless. That’s not always true. I’ve rubbed camphor-rich balms on sore muscles after a tiring day chopping wood, and my skin got red and irritated. Camphene causes that same reaction in some users. Clinical studies show camphene can irritate skin, nose, and eyes. Some folks notice their skin tingling or burning—sometimes worse for people who already have sensitive skin or eczema.
Breathing in camphene vapors can sometimes sting your nasal passages or make your chest feel tight. Asthmatics or those with chronic lung issues could see their symptoms flare up if they’re around essential oils with camphene. I’ve sat in crowded wellness workshops where diffusing too much of anything set off coughing and watery eyes, but camphene-rich blends were especially noticeable.
Digestive Troubles and Potential Toxicity
A neighbor once tried ingesting a homemade camphene tincture after reading a trendy online article about its health “benefits.” It hit her hard. She felt sick, crampy, and nauseous. Swallowing camphene can lead to stomach pain and vomiting, even at doses lower than you’d guess, and much larger amounts could possibly mess with the nervous system, causing confusion or seizures. The National Institutes of Health warns that swallowing products with camphene in high concentrations is risky, so you want to keep these mixtures locked up and away from curious kids.
Allergy Risks and Sensitization
I’ve heard stories from folks in long-term care homes who got rashes or headaches after new cleaning sprays were introduced. Camphene appears as an ingredient in some air fresheners and cleaning products, and repeated skin contact can set off allergies over time. Some research points to camphene acting as a sensitizer—meaning allergies get worse the more you’re around it. More than a few people have ended up at the doctor, not realizing the stuff in their lotion started the problem.
How Can You Protect Yourself?
It pays to take camphene seriously and treat it like any chemical—natural or not. Read the product labels, especially if you’re prone to breathing troubles or allergic reactions. Do a patch test before slathering on anything new. If you work in a job—like cleaning, aromatherapy, or health care—where you handle lots of essential oil products, swap stories with coworkers and keep up with workplace safety guidelines. Gloves and plenty of ventilation go a long way in preventing problems. If you notice itching, trouble breathing, or dizziness, set the camphene aside and see a healthcare provider who knows what to look for. This common terpene packs a punch, and staying aware prevents a lot of unnecessary trouble.
Understanding Camphene’s Nature
Camphene shows up clear or white, with a strong odor that’s hard to miss. Anyone who has handled it can’t forget the distinct smell that lingers on gloves or bench tops. Packed inside this little molecule, there’s a powerful ability to catch fire. A spark, a stray flame in the wrong place—suddenly, things can get dangerous. Chemical storage fails the moment someone stores this material like it’s just another solvent. Too many stories run through labs and warehouses about near misses or worse.
Fire Safety Takes Priority
Working hands-on in production sites and chemistry labs, I’ve watched people learn—often too late—how important it is to separate flammable substances. Camphene should always rest in a cool space, well away from anything heat-producing. No one stores a chemical like camphene next to heat lamps, steam pipes, or sunny windows. It attracts risk. A dedicated flammable cabinet, with double-walled steel construction, keeps it out of harm’s way and buys precious time if a fire breaks out. Bright red “Flammable” stickers on the door signal even to tired eyes that this isn’t just extra glassware or old files.
Sealed Containers and Chemical Compatibility
Strong, airtight containers matter, since fumes escape easily. I’ve seen old plastic caps warp or crack, so I stick to original packaging or choose glass containers rated for chemicals. Camphene attacks soft plastics and rubber, which can swell or degrade, so nothing beats metal or glass. Honest labeling stops mistakes. During inventory season, unlabeled jars invite confusion—and risk someone mixing incompatible chemicals without realizing it. Bad things happen when camphene leaks and mixes with acids or oxidizers.
Ventilation and Temperature
Camphene evaporates faster than water spills on a hot plate. Heavy vapors hug the ground, collecting in low spots. Good ventilation keeps air moving, pushing fumes toward exhaust fans and away from workers’ lungs. In a stuffy storeroom, vapor buildup spells trouble—an ignition source turns a quiet day into a disaster. Experience taught me to treat temperature as just as important as ventilation. Nothing beats a spot where the temperature never climbs much above room level. Exposure to cold doesn’t pose the same threat as heat does for camphene, but avoiding frost keeps containers from cracking.
Securing Against Spills and Leaks
Even careful people spill things. Lining shelves with spill trays catches leaks before they run out of sight. Cleanup supplies should sit within reach, not boxed away at the end of a dark hallway. If a bottle breaks, having absorbent pads and a plan ready saves time, nerves, and possibly lives. In my own work, making up spill kits with instructions—easy to read, posted in plain sight—helped new staff avoid panic.
Training People to Handle Camphene
A safe storage area means nothing if hands go in untrained. Bringing new folks into the fold, I explain what can go wrong, using real examples instead of dry bullet points. Repeated practice beats a dusty safety binder. PPE like goggles and chemical-resistant gloves stay mandatory, no matter how many years someone’s worked with flammable chemicals. What matters most: a team that knows the risks and respects them, keeping camphene storage as safe as the precautions allow.
Looking At Where Camphene Comes From
Camphene shows up in everyday life without much fanfare. You’ll find it listed in the fine print of fragrance bottles, cleaning sprays, and sometimes in dietary supplements. The real curiosity is whether this stuff comes straight from nature or a lab beaker. It’s not a black-and-white story—manufacturers pull camphene from two very different playbooks.
Plants like turpentine trees, sage, and fir give us camphene the old-fashioned way. In these settings, camphene gets made inside the plant as part of the wild chemistry sessions that keep leaves green and bugs at bay. Workers harvest the raw plant, run it through distillation, and the camphene separates out along with other essential oils. You’ll catch this version in many natural essential oil blends—what folks call “plant-derived.”
The Synthetic Route
For every bottle that boasts “from the forest,” there's a factory just outside a city where chemists turn pinene—a terpene from turpentine—into camphene through acid-catalyzed isomerization. It’s fast, predictable, and works year-round, regardless of drought or blight. Since global demand for camphene keeps climbing, especially in the flavor, fragrance, and rubber industries, the lab-made approach now fills most of the world’s orders.
A lot of folks assume “synthetic” means something ominous. The truth is, synthetic camphene comes from organic starting materials like pine oil. The labs break apart molecules and rearrange them, basically mimicking what nature does, only on a much larger scale. A report by the International Fragrance Association points out that without synthetic camphene, supply chains would choke and prices would swing all over the map. That’s especially true after freak weather knocks out forest harvests.
Why It Matters to Ask
This question doesn’t land in a philosophy classroom. It surfaces at farmer’s markets, supplement stores, and in regulatory offices across the world. Many consumers want ingredients with clear sources and simple stories. Some folks feel better trusting plant-derived products. Others might have allergies or support sustainability, which complicates the choice.
On the flip side, purity and consistency steer many toward synthetic options. Natural camphene batches sometimes carry trace impurities depending on the plant’s growing season, soil, and the distillation method. Synthetic camphene can promise a tidier chemical profile. Long-term inhalation or ingestion brings health concerns, too. The European Chemicals Agency includes camphene on lists for precaution, but routine levels found in foods or scents remain well below the safety threshold.
Sorting Fact From Fiction
It’s easy to be swept up by marketing that paints one version as pure and safe, the other as risky or fake. Look under the hood, and both natural and synthetic camphene rely on chemistry, whether in forests or factories. Quality comes down to sourcing, transparency, and testing. My experience dealing with essential oil suppliers tells me that true safety comes from honest labeling and third-party verification, not just where something grows.
For anyone buying products with camphene, checking ingredient sourcing policies helps. Companies willing to share lab results and sourcing details set themselves apart. Regulatory agencies in the US, EU, and Asia require rigorous safety reviews and regular spot checks. This level of oversight means what shows up on shelves lines up with established safety guidelines.
Looking Ahead
Stronger consumer interest in supply chain transparency makes it more likely that labels on everyday goods will continue to spell out whether ingredients are plant-based or lab-made. People deserve to know the backstory of what goes into their homes or onto their skin. Both versions of camphene have a place in today’s market—it ultimately comes down to making informed choices and letting science guide those calls.
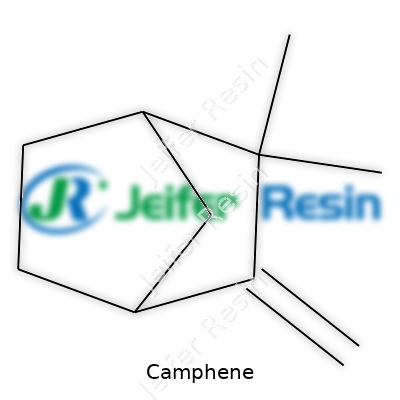
| Names | |
| Preferred IUPAC name | 2,2-dimethyl-3-methylidenebicyclo[2.2.1]heptane |
| Other names |
2,2-Dimethylethylene
2,2-Dimethyl Ethylene Isobornylene p-Menthene |
| Pronunciation | /ˈkæm.fiːn/ |
| Identifiers | |
| CAS Number | 79-92-5 |
| Beilstein Reference | 1281344 |
| ChEBI | CHEBI:28683 |
| ChEMBL | CHEMBL16240 |
| ChemSpider | 54640 |
| DrugBank | DB03606 |
| ECHA InfoCard | 06af12d8-a027-4179-8367-164180b6c3c2 |
| EC Number | 204-313-9 |
| Gmelin Reference | Gm. 285 |
| KEGG | C06574 |
| MeSH | D002182 |
| PubChem CID | 6616 |
| RTECS number | GD0875000 |
| UNII | SK72B10SIF |
| UN number | UN2710 |
| Properties | |
| Chemical formula | C10H16 |
| Molar mass | 136.24 g/mol |
| Appearance | Colorless liquid with a pungent odor |
| Odor | Pungent, penetrating |
| Density | 0.842 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.8 |
| Vapor pressure | 0.8 mmHg (25 °C) |
| Acidity (pKa) | Approximately 41 |
| Basicity (pKb) | Camphene has a pKb of 10.89 |
| Magnetic susceptibility (χ) | -74.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4600 |
| Viscosity | 1.59 cP (25°C) |
| Dipole moment | 0.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 355.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -444.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3192.2 kJ/mol |
| Pharmacology | |
| ATC code | D03AX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07, Dgr, H226, H315, H317, H319 |
| Pictograms | Flame, Exclamation mark |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H410 |
| Precautionary statements | P210, P261, P273, P280, P301+P312, P302+P352, P305+P351+P338, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-4-2 |
| Flash point | Flash point: “38°C” |
| Autoignition temperature | 385 °C |
| Explosive limits | 0.8% - 6.7% |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5 g/kg (rat, oral) |
| NIOSH | GV0525000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Camphene: "Not established |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Camphor
Borneol Isoborneol Pinene Thujene |



